Hemophilia gene therapy represents a groundbreaking advance in the treatment of this lifelong bleeding disorder, providing hope to those who manage its challenges daily. For patients like Terence Blue, the introduction of treatments such as Hemgenix marks a significant milestone, potentially alleviating the need for routine clotting factor injections. With FDA approval granted in 2022, this innovative approach aims to address the genetic root of hemophilia, demonstrating promising advancements in hemophilia care and offering patients new ways to engage with life. As gene therapy for hemophilia continues to evolve, it raises excitement not only for the possibilities it holds but also for the potential to fundamentally change how individuals live with hemophilia. By harnessing the power of modern science, these new treatments also emphasize the growing landscape of gene therapy advancements that may redefine patient outcomes in the years to come.
Innovative approaches to treating hemophilia have ushered in a new era of possibilities for individuals affected by this condition. Recent developments in gene-based therapies, specifically tailored for managing hemophilia, show encouraging outcomes for patients looking for alternatives to traditional treatment methods. For instance, the Hemgenix therapy has emerged as a beacon of hope, providing effective treatment details that outline a path towards greater quality of life for those navigating the complexities of hemophilia. As the medical field embraces new treatments for hemophilia, the impact of gene therapy resonates with the aspirations of patients trying to lead healthier, freer lives. Ultimately, these advancements carry the potential to transform the landscape of hemophilia care, underscoring the importance of ongoing research and development in gene therapy.
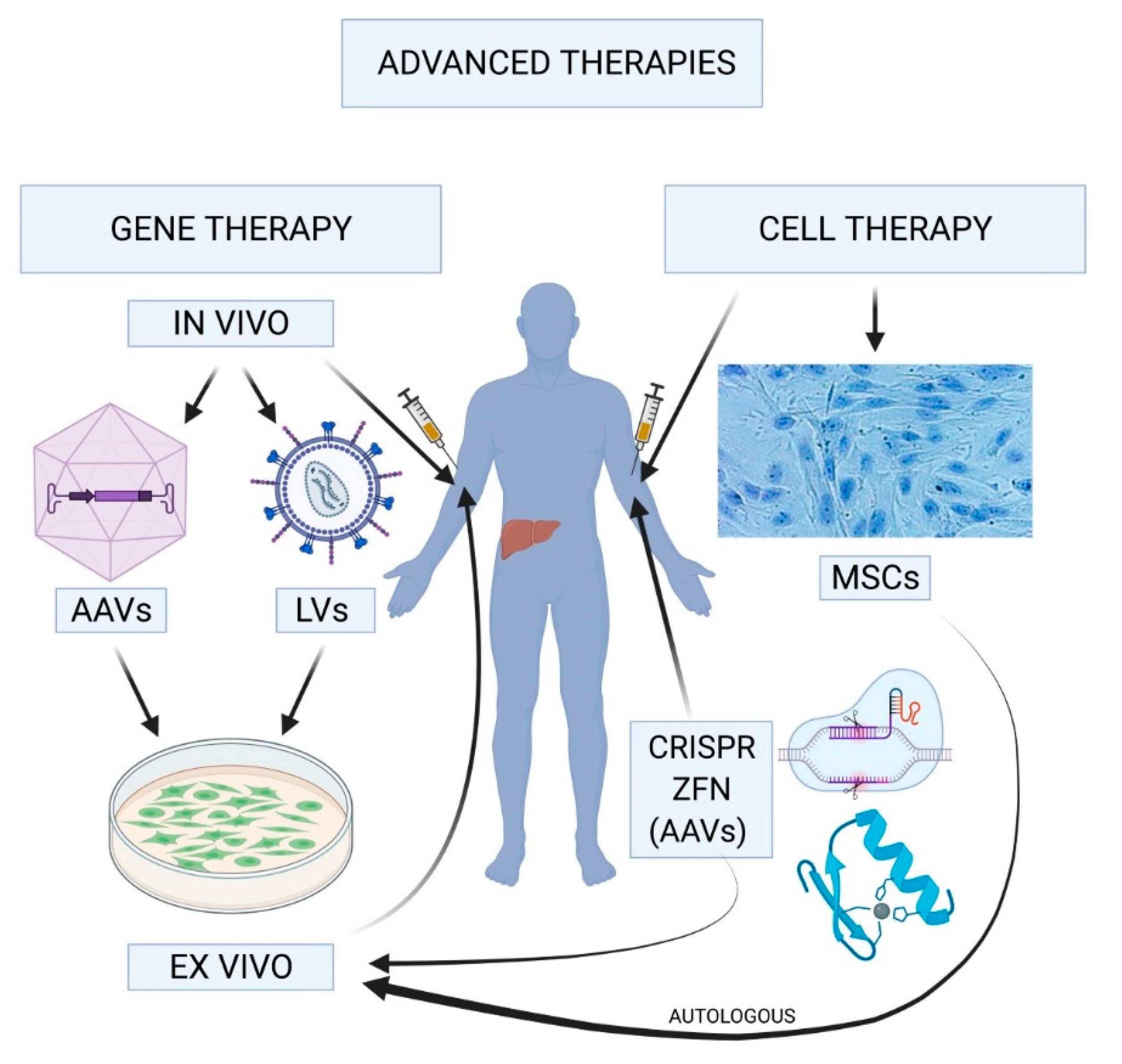
Understanding Gene Therapy for Hemophilia
Gene therapy for hemophilia is a groundbreaking approach that aims to address the root cause of the condition rather than merely managing its symptoms. Traditional treatments involve regular infusions of clotting factors, but gene therapy, such as Hemgenix, seeks to introduce a corrected version of the faulty gene responsible for hemophilia. By effectively altering the genetic instructions in a patient’s liver cells, this treatment has the potential to produce adequate levels of clotting factor IX, significantly improving a patient’s quality of life. With advancements in technology, gene therapy has become increasingly viable, offering hope for long-lasting relief and reducing the need for frequent needle-based treatments.
Living with hemophilia has historically posed significant challenges, including the constant fear of bleeding and the burden of maintaining regular intravenous treatments. By employing gene therapy, patients like Terence Blue are witnessing a paradigm shift in their treatment. Instead of worrying about spontaneous bleeds or the discomfort associated with regular shots, they can now anticipate a future where their need for prophylactic infusions may be dramatically reduced. This transformation represents not just medical advancement, but a profound psychological relief for many, as they navigate life with less anxiety and greater freedom.
Hemgenix Treatment Details and Benefits
Hemgenix, the first approved gene therapy for hemophilia B, was developed to offer a one-time solution for patients suffering from this lifelong condition. Its administration utilizes a specially modified virus that targets liver cells, where it delivers the corrected gene responsible for clotting factor production. Patients receive the treatment through a simple outpatient infusion, drastically changing the landscape of hemophilia management. For individuals like Terence Blue, whose lives have been dominated by regular injections, this therapy symbolizes both hope and healing, making living with hemophilia much more manageable.
The benefits of Hemgenix extend beyond just the elimination of needles. Clinical studies have shown that many patients who received the therapy not only saw a rise in their factor IX levels but also reported fewer bleeds and increased physical activity. By nursing a significant reduction in the frequency of infusions, patients reclaim hours that would typically be spent in medical facilities. These outcomes highlight the potential of new treatments for hemophilia, promising a future where individuals can lead more active, fulfilling lives without constant medical interventions.
Life After Gene Therapy: Living with Hemophilia
For those living with hemophilia, the journey doesn’t end with gene therapy. Adjusting to life after receiving treatments like Hemgenix involves learning to trust one’s body again and managing the psychological aspects of the condition. Many patients have shared that the prospect of living with fewer disruptions caused by spontaneous bleeds or the anxiety of needing immediate medical attention has changed their outlook on life. They can engage in activities that were previously daunting or off-limits, which significantly improves not just their physical health but also their mental well-being.
This newfound freedom, however, also comes with challenges. Individuals who have undergone gene therapy, like Terence Blue, often have to navigate their identities as hemophilia patients while adapting to a new norm. They may find themselves in social situations where they need to educate friends or family about their condition and the implications of their treatment, which can still present emotional hurdles. Understanding that living with hemophilia is still part of their identity, even post-treatment, is crucial in their ongoing journey toward holistic health.
Advancements in Hemophilia Treatments
The landscape of hemophilia treatment is evolving rapidly, with advancements in gene therapy marking significant milestones in patient care. As more therapies like Hemgenix gain approval, they provide a glimpse into a future where hemophilia can be managed more effectively, reducing the frequency and severity of bleeds. Research and clinical trials continue to uncover new methods of harnessing gene therapy, ensuring that patients have access to cutting-edge solutions that could change their lives for the better.
However, the introduction of these new treatments brings forth unique challenges, particularly related to pricing and patient access. With therapies costing millions, like the $3.5 million price tag for Hemgenix, discussions around insurance coverage, market acceptance, and equitable access have come to the forefront. Ensuring that patients gain timely access to these innovations while addressing economic realities will be critical as the field of hemophilia treatment continues to advance.
The Future of Gene Therapy Advancements
As the field of gene therapy for hemophilia advances, the potential for future solutions seems brighter than ever. Researchers are continuously working to refine the technologies used in these therapies, improving delivery methods, safety profiles, and overall effectiveness. With ongoing trials and studies, there’s optimism about expanding gene therapy applications not just for hemophilia but for a range of genetic disorders. This innovation could revolutionize how we approach hereditary conditions, making long-term management simpler and more effective.
Furthermore, the integration of patient feedback into the development process is becoming increasingly important. Understanding the real-world experiences of individuals living with hemophilia can provide invaluable insights that drive further enhancements in therapy design and delivery. The drive for patient-centered care underscores a commitment to not only advancing medical science but also improving quality of life for those affected by these conditions. With continued investment and focus on research, the future of gene therapy in treating hemophilia holds tremendous promise.
Market Considerations for Gene Therapies
Despite the enormous potential of gene therapy to transform the treatment landscape for hemophilia, market dynamics can complicate its implementation. Drug pricing, insurance negotiations, and patient acceptance are paramount issues that can impact the availability of treatments like Hemgenix. For instance, while the therapy has been shown to significantly improve patient outcomes, the high cost can deter both patients and healthcare providers, leading to difficult choices regarding care.
Moreover, the withdrawal of marketing authorization for other hemophilia treatments indicates an urgent need for affordable solutions that meet market demands. As healthcare providers and researchers strive to bring effective therapies to patients, addressing the economic disparities in accessing these treatments remains crucial. The challenge lies in balancing innovation with realistic market conditions to ensure that life-changing therapies reach those who need them most.
Patient Experiences with Hemgenix
Patients like Terence Blue have become living testimonies to the transformative effects of Hemgenix and similar gene therapies. Blue’s narrative highlights not only his initial apprehensions about the therapy but also the sense of relief and newfound hope that come with experiencing tangible improvements in his condition. Many patients share akin stories of anticipating life with fewer medical constraints, enhancing their social engagements and overall quality of life.
As more individuals receive gene therapies, gathering and analyzing patient experiences can offer crucial insights into the treatment’s effectiveness over time. With each success story, the motivation to advance research and development grows stronger. Septuagenarians advocating for support networks and communities can also help shatter the stigma surrounding hemophilia, promoting a more inclusive environment for individuals living with this condition.
The Role of Clinical Trials in Advancing Treatments
Clinical trials are at the heart of developing new treatments for hemophilia, providing essential data that helps researchers understand the safety and effectiveness of therapies like Hemgenix. These trials not only aim to demonstrate the potential benefits of gene therapies but also explore their long-term implications for patients. Participation in clinical trials can offer patients early access to cutting-edge treatments while contributing to the broader scientific knowledge that paves the way for future advancements.
Moreover, clinical trials can help uncover challenges associated with new therapies, prompting necessary adjustments and refinements. They create a feedback loop that informs ongoing research and development to ensure that patient needs remain at the forefront of medical progress. By actively engaging patients in the research process, the medical community can build a more comprehensive understanding of hemophilia, moving ever closer to optimized treatment solutions that enhance patient lives.
Navigating Life with Modern Hemophilia Treatments
As patients transition to modern treatments like Hemgenix, navigating their new realities can present both opportunities and challenges. Patients must develop coping strategies to adapt to their evolving health status while continuing to monitor their overall wellness. This might involve engaging with healthcare providers regularly, understanding the nuances of their condition post-treatment, and forming supportive relationships within the hemophilia community for shared experiences and insights.
Adjusting to life after gene therapy may also include reevaluating personal goals, hobbies, and even career aspirations, as the burdens of constant treatment diminish. Engaging in new activities or pursuing previously set-aside passions can play an essential role in reclaiming a fulfilling life post-treatment. This shift emphasizes the importance of holistic care, considering physical, emotional, and social aspects of health as patients redefine what it means to live with hemophilia in a new era of medical possibilities.
Frequently Asked Questions
What is hemophilia gene therapy and how does it work?
Hemophilia gene therapy, such as the FDA-approved Hemgenix for hemophilia B, involves inserting a corrected copy of the mutated gene responsible for hemophilia into the patient’s liver cells. This gene therapy aims to enable the body to produce the missing clotting factor IX, reducing the need for regular infusions of clotting factors and decreasing bleeding incidents.
What are the benefits of gene therapy for hemophilia over traditional treatments?
Gene therapy for hemophilia, like Hemgenix, offers significant benefits compared to traditional treatments. Patients can potentially reduce or eliminate the need for frequent clotting factor injections, leading to a more normal lifestyle. It addresses the underlying genetic cause of hemophilia, aiming for long-term or even permanent effects, instead of just managing symptoms.
What are the advancements in gene therapy for hemophilia?
Recent advancements in gene therapy for hemophilia include the development of Hemgenix, which was FDA-approved in late 2022. This innovative treatment uses a virus to deliver a normal copy of the gene responsible for clotting factor production directly to liver cells, greatly improving the safety and effectiveness of hemophilia management.
What does the Hemgenix treatment process involve for patients?
The Hemgenix treatment process involves an outpatient infusion where the patient receives the gene therapy through an intravenous line. After the treatment, patients are monitored for a few hours. The therapy aims to integrate into the patient’s liver cells, allowing for the production of factor IX, which is deficient in hemophilia B.
How has living with hemophilia changed with new treatments like Hemgenix?
Living with hemophilia has significantly improved with new treatments like Hemgenix. Patients now have the opportunity to live a life with fewer disruptions from frequent injections and the anxiety of bleeding episodes. The potential for a long-lasting solution through gene therapy provides hope and encourages a more active lifestyle for those affected.
What should patients consider before opting for hemophilia gene therapy like Hemgenix?
Patients considering hemophilia gene therapy, such as Hemgenix, should evaluate factors like the treatment’s cost, potential side effects, and long-term efficacy. Consulting with healthcare providers about individual health conditions and reviewing data from clinical trials can help inform their decision regarding this groundbreaking form of treatment.
Are there any risks associated with gene therapy for hemophilia such as Hemgenix?
While gene therapy for hemophilia, like Hemgenix, shows promising results, it does carry potential risks, including immune reactions to the viral vector used in treatment, liver enzyme elevation, and uncertainties about long-term effects. Patients should discuss these risks thoroughly with their medical team before proceeding.
What is the long-term outlook for patients treated with hemophilia gene therapy?
The long-term outlook for patients treated with hemophilia gene therapy is optimistic. Clinical studies have shown that a significant percentage of patients treated with Hemgenix continue to maintain adequate factor IX levels and do not require regular infusions for years after treatment, marking a potential shift towards a more manageable lifestyle.
How does gene therapy for hemophilia compare to future treatments in development?
Gene therapy for hemophilia, particularly advancements like Hemgenix, represents a significant leap forward compared to traditional treatments and other potential future therapies. The ongoing research in gene and cell therapy is broadening options, promising new avenues to potentially cure or better manage various genetic conditions like hemophilia.
What are the implications of gene therapy advancements for patients living with hemophilia?
The advancements in gene therapy for hemophilia suggest a transformative impact on patients’ lives. By potentially eliminating the need for frequent treatments and significantly reducing bleeding risks, gene therapies could lead to improved quality of life, decreased treatment burden, and increased hope for a future without hemophilia-related limitations.
| Key Point | Details |
|---|---|
| Introduction to Hemophilia Gene Therapy | Terence Blue was the first patient in New England to receive Hemgenix, a novel gene therapy for hemophilia B. |
| Life with Hemophilia | Blue has managed hemophilia his whole life, requiring regular injections of clotting factor to prevent bleeding. |
| Advancements in Treatment | Synthetic factors have improved treatment safety and convenience, but gene therapy represents a potential cure. |
| The Approval of Hemgenix | Hemgenix was developed by CSL Behring and received FDA approval in November 2022. |
| Market Challenges | High costs and market pressures may affect the availability and acceptance of gene therapies. |
| Outcomes of Hemgenix | After treatment with Hemgenix, Blue’s factor IX levels improved significantly, lowering his dependency on injections. |
| Impact on Quality of Life | Blue experienced faster healing and reduced worries about bleeding, significantly improving his quality of life. |
Summary
Hemophilia gene therapy represents a groundbreaking advancement in treatment options, offering patients like Terence Blue new hope for managing their condition. With promising results from therapies like Hemgenix, individuals may experience improved health outcomes, reduced reliance on regular injections, and an enhanced quality of life. This innovation signifies a major step forward in the journey towards curing hemophilia, heralding a brighter future for those affected by this challenging genetic disorder.