Medical research safety is a cornerstone of ethical clinical trials, ensuring that the rights and well-being of participants are upheld throughout the research process. With an increasingly complex landscape shaped by funding cuts in medical studies, the integrity of patient safety in research is at risk. The halt in federal support, particularly from the National Institutes of Health (NIH), has significant implications, as it jeopardizes the essential oversight provided by Institutional Review Boards (IRBs). These bodies play a crucial role in maintaining clinical trial ethics, meticulously evaluating research proposals to mitigate risks and protect participants. As we delve into the ramifications of this funding freeze, it becomes clear that the impacts are profound, extending beyond individual studies to the larger framework of medical ethics and public trust.
Ensuring the safety and rights of individuals participating in medical research is of paramount importance, with systems in place to uphold these standards. The concept of participant protection encompasses various aspects, including the oversight of clinical studies and the ethical considerations involved in patient recruitment and consent. In light of recent challenges, such as decreased financial backing for scientific inquiries, the potential ramifications on human subject safety cannot be overlooked. The role of regulatory agencies and oversight committees becomes more critical in navigating the intricate ethics of clinical trials amidst external pressures. A concerted effort is required to maintain the delicate balance between advancing research and safeguarding the individuals who contribute to medical breakthroughs.
The Impact of Funding Cuts on Patient Safety in Medical Research
Funding cuts in medical research can have a profound effect on patient safety and the ethical conduct of clinical trials. When financial resources are restricted, it not only hampers the ability to conduct robust studies but also affects the oversight processes necessary to safeguard participants’ rights and welfare. With fewer funds available, Institutional Review Boards (IRBs) may struggle to perform comprehensive reviews and monitoring of ongoing trials. Consequently, this can lead to lapses in patient safety measures, reducing the overall quality and integrity of the research that is vital for future medical advancements.
Moreover, the halting of federal grants, such as those seen recently for Harvard’s research programs, creates significant disruptions in ongoing studies. This discontinuation can prevent necessary audits and assessments that are crucial for evaluating participant safety historically and currently. Each study relies on adequate funds to ensure that all ethical considerations and safety protocols are upheld. In the absence of sufficient funding, researchers might be forced to cut corners or delay essential procedures, potentially putting participant safety at risk.
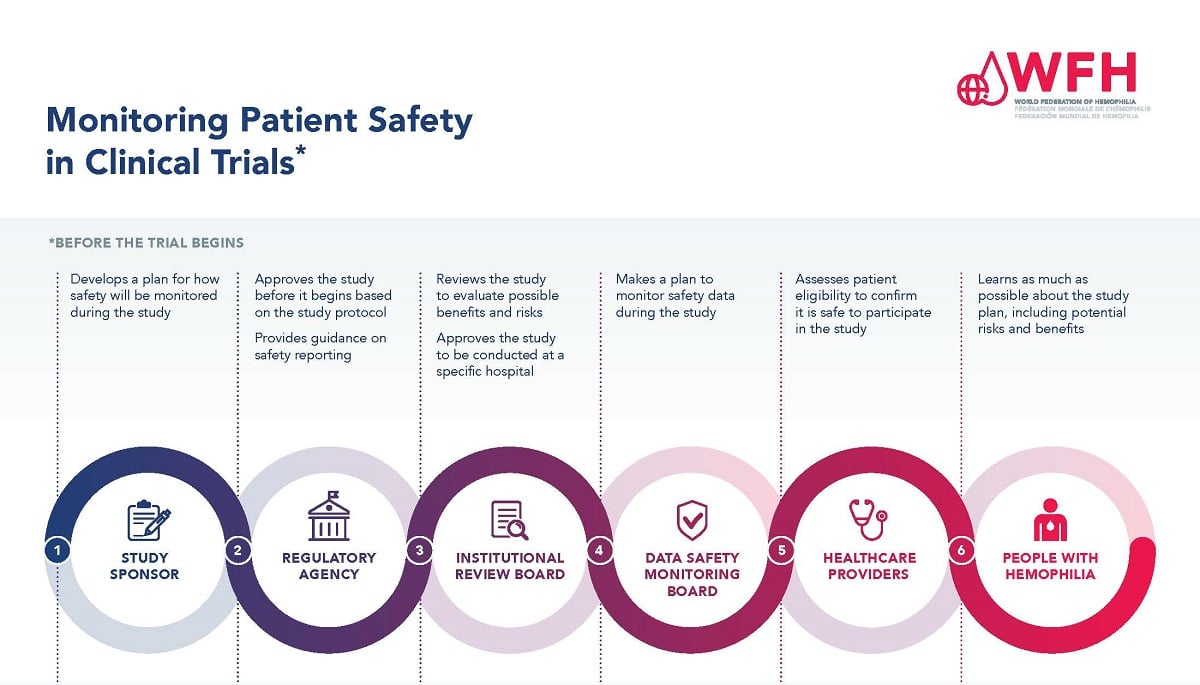
Role of Institutional Review Boards in Ensuring Research Ethics
Institutional Review Boards (IRBs) serve as the cornerstone of patient safety in clinical research. They are tasked with meticulously vetting study proposals to assess risks against potential benefits, ensuring that research activities comply with established ethical standards and regulations. IRBs evaluate informed consent processes, facilitate post-study safety monitoring, and oversee the ethical treatment of participants throughout the research cycle. Their role is critical in mitigating adverse effects that could arise during clinical trials, thereby upholding the integrity of the research process.
Furthermore, IRBs act as a liaison between researchers and various regulatory bodies, helping to navigate the landscape of compliance and oversight. This includes interfacing with funders like the NIH, which significantly influences research safety through its funding policies. In light of recent funding cuts, the risk posed to patient safety increases because fewer resources mean that IRBs may lack the manpower and expertise necessary to thoroughly review and oversee studies. This potentially jeopardizes the rights and safety of the very individuals that research aims to help.
Consequences of Halted Research on Clinical Trials
The suspension of medical research not only affects the research institutions involved but also has tangible consequences for participants enrolled in clinical trials. When studies are abruptly halted due to funding cuts, participants are left in uncertain positions, often without clarity on their ongoing involvement or how their health could be impacted. Moreover, the disruption can sow seeds of mistrust in the research community, particularly among demographics that already harbor skepticism towards clinical trials due to historical injustices.
As these disruptions continue, they can lead to a decrease in participant recruitment and retention, making it challenging to conduct studies that address pressing health issues. For example, when collaborative research among multiple sites is interrupted, as illustrated by the recent challenges faced by the SMART IRB program, this limits the diversity of patient perspectives and experiences considered in research studies. Such limitations not only compromise the quality of research outcomes but also hinder advancements in treatments thereby threatening public health at large.
The Need for Comprehensive Ethics in Medical Research
The ethical landscape of medical research is continually shaped by historical events that highlighted the necessity for strict oversight, such as unethical practices observed during the Tuskegee Study and Nazi medical experiments. These experiences underscored the importance of informed consent and the protection of vulnerable populations in research. Today’s IRBs are essential in ensuring that these ethical principles are embedded in every aspect of research design and implementation, fostering public confidence in scientific inquiry.
As funding becomes increasingly restricted, the capacity of IRBs to enforce these ethical standards is at risk. It raises critical questions about the sustainability of patient protections in an environment where research funding is critical. The integration of rigorously enforced ethical guidelines must remain a priority, especially in federally funded studies. Ensuring a commitment to ethical research practices is vital not only for protecting patient interests but also for maintaining the credibility of the medical research field in the face of current challenges.
Challenges Faced by Researchers Amidst Funding Cuts
Researchers are encountering unparalleled challenges due to the current funding cuts, which threaten the stability and advancement of medical research. With diminishing NIH research funding and other federal support, many institutions find themselves at a crossroads where they must decide whether to dramatically scale back their projects or seek alternative funding sources. This situation can deter innovative research pursuits, especially in fields requiring extensive resources and long-term investment.
Additionally, funding cuts can disproportionately impact emerging researchers and smaller institutions that rely heavily on federal grants to conduct their studies. Without the ability to obtain necessary research support, the academic landscape risks losing some of its brightest minds who are eager to contribute to the advancement of health sciences. The ripple effects of these financial constraints extend beyond individual researchers, potentially impacting healthcare outcomes and the development of new therapies for patients who rely on continued innovation in medical research.
Preserving Patient Trust in Clinical Trials
Maintaining patient trust is paramount for ensuring successful participation in clinical trials. However, recent funding cuts and the subsequent upheaval in research operations risk eroding this trust. When trials are halted or when IRBs cannot provide the necessary oversight, prospective and current participants may lose confidence in the ethical integrity of research studies. This erosion of trust can have lasting impacts, leading individuals to avoid participating in clinical trials altogether, thus diminishing the quality of the research outcomes.
To rebuild and preserve this trust, it is critical for institutions to communicate transparently with potential participants about their commitments to safety and ethical oversight, especially in light of funding uncertainties. Efforts should focus on reaffirming the ethical standards that guide clinical research and demonstrating a commitment to participant welfare at all times. Trust can only be reestablished through consistent, responsible actions that prioritize participant safety and showcase the benefits of participating in research for the advancement of medical knowledge.
Future of Medical Research Amidst Financial Uncertainty
The future of medical research hinges on navigating the current financial uncertainty while upholding the commitment to ethical patient care. As funding cuts persist, it is essential for academic and research institutions to explore innovative funding solutions, including private partnerships, community funding, and alternative grant sources. These alternatives could help alleviate some pressures that funding cuts impose and sustain vital research initiatives.
Moreover, embracing a multidisciplinary approach that fosters collaboration among hospitals, universities, and the pharmaceutical industry can also pave the way for new funding avenues. This collaborative spirit will not only enhance the quality of research conducted but it can help ensure that patient safety remains a top priority amidst challenging economic landscapes. Ultimately, it is through perseverance, innovation, and commitment to ethical research practices that the medical research community can overcome these obstacles and continue to improve health outcomes for patients.
The Role of Government in Supporting Research Ethics
The government plays a pivotal role in shaping the landscape of research ethics through funding and regulation. By providing necessary resources through agencies like the NIH, the government ensures that research institutions can implement ethical practices and conduct thorough oversight of clinical trials. However, recent trends in funding cuts demonstrate a critical need for a re-evaluation of how these agencies can better support IRBs and other regulatory frameworks to maintain ethical standards across all levels of medical research.
Additionally, the government’s commitment to preserving patient safety and promoting ethical research should be reflected in policies that prioritize funding for studies that address pressing healthcare needs. Implementing regulations that require explicit ethical compliance checks in funding proposals could also serve as a mechanism to strengthen oversight and accountability in research practices. Ultimately, a collaborative approach among government, research institutions, and the public will be essential to sustain ethical standards in medical research.
Advocating for Patient-Centered Research
Amid rising funding cuts and increased scrutiny of research methodologies, advocating for patient-centered research has never been more critical. This approach emphasizes the importance of involving patients in the design and implementation of studies, ensuring that their perspectives help shape research questions and priorities. By prioritizing patient engagement, researchers can enhance the relevance of their studies and improve participant trust and safety.
Moreover, patient-centered research aligns with ethical standards that dictate respect for participants’ rights and dignity. It encourages transparency and informed consent throughout the research process, key elements needed to uphold ethical obligations in medical studies. As advocates for patient safety, researchers must strive to create environments that not only welcome but actively engage patient voices, thereby enriching the research landscape and reinforcing public trust.
Frequently Asked Questions
What is the importance of patient safety in research?
Patient safety in research is crucial as it ensures that the rights and welfare of participants are prioritized throughout any study. Institutional Review Boards (IRBs) play a vital role in this process by reviewing and approving research protocols to mitigate risks, ensure informed consent, and monitor ongoing research activities, thus safeguarding participants’ health and safety.
How do funding cuts in medical studies affect patient safety?
Funding cuts in medical studies can severely disrupt processes that ensure patient safety. Such reductions can lead to the suspension of research projects, insufficient oversight by IRBs, and decreased resources for monitoring adverse events, ultimately compromising the safety and well-being of study participants.
What is the role of IRB oversight in medical research safety?
IRB oversight is fundamental for medical research safety as it establishes protocols that protect participants. By thoroughly reviewing research proposals, ensuring informed consent, and monitoring studies for ethical compliance, IRBs uphold the integrity of trials and help prevent potential harm to participants.
How does NIH research funding contribute to medical research safety?
NIH research funding is integral to medical research safety as it supports rigorous IRB review processes and regulatory compliance. These funds help researchers implement ethical standards for studies involving human subjects, protecting participants and enhancing overall research quality.
What are the ethical considerations in clinical trials that impact patient safety?
Ethical considerations in clinical trials include ensuring informed consent, risk assessment, and background checks of participants. These factors directly impact patient safety by requiring that trials minimize risk, maximize benefits, and provide participants with necessary information to make informed choices about their involvement.
How do historical events influence current practices in patient safety during research?
Historical events, such as the Tuskegee syphilis study, have profoundly influenced current practices in patient safety. These events highlighted the critical need for oversight and ethical guidelines in research, leading to the establishment of IRBs and comprehensively designed protocols that prioritize patients’ rights and safety.
What are the consequences of halting medical research funding on patient safety?
Halting medical research funding can lead to the disruption of ongoing studies, which may stop safety monitoring and patient oversight. This not only jeopardizes participant safety but also fosters public mistrust in research methodologies, which can deter future participation and cooperation among communities.
Why is it essential for medical research studies to implement a single IRB (sIRB) for multi-site research?
Implementing a single IRB (sIRB) for multi-site research streamlines the review process, ensuring consistent oversight and faster approval while maintaining high standards for patient safety. This approach reduces redundancy, minimizes delays, and enhances collaboration across institutions, ultimately benefiting participants in clinical trials.
| Key Points | Details |
|---|---|
| Funding Cuts Impact | The Trump administration’s freeze of over $2 billion in research grants to Harvard disrupts safety efforts for patients participating in studies. |
| Role of IRBs | Institutional Review Boards (IRBs) ensure participants’ rights and welfare, reviewing research proposals to protect study subjects. |
| Importance of SMART IRB | SMART IRB facilitates oversight for multisite studies, preventing delays and promoting collaborative research. |
| Risks of Halting Research | Halting research studies can reinforce public mistrust and skepticism, burdens participants and disrupt collaborative efforts. |
| Historical Context | Past medical experiments highlight the necessity for oversight systems, fostering the establishment of ethical research review processes. |
Summary
Medical research safety is crucial for protecting participants, especially in light of recent funding cuts that threaten oversight systems. These cuts disrupt collaborative efforts and vital protections integrated by IRBs, which serve to ensure that studies are conducted ethically and participants’ rights are enforced. Historical abuses in research underscore the ongoing need for stringent safety regulations. Therefore, maintaining robust funding for medical research oversight is essential not only for participant protection but also for public confidence in clinical studies.